Krystal leverages its proprietary platform to develop novel, redosable gene therapies for diseases with a high unmet medical need.
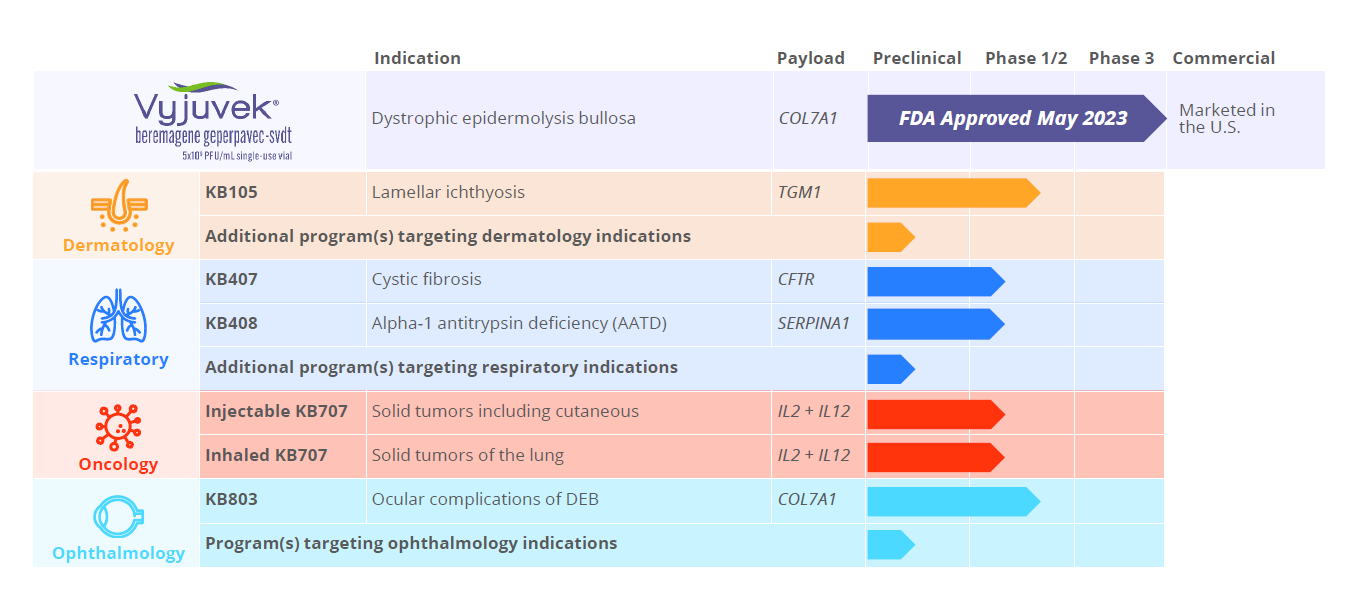
Krystal Pipeline
Near-term focus on three target tissues with additional pipeline expansion under investigation.
Jeune Aesthetics Pipeline
Jeune Aesthetics, a wholly-owned subsidiary of Krystal Biotech, uses the same platform to treat aesthetic skin conditions.

Not an actual patient

Get more information on Jeune Aesthetics, Inc.
Discovering, developing, and commercializing genetic medicines with purpose
Krystal Pipeline



 United States
United States Germany
Germany France
France Japan
Japan